

In short, because you're adding protons, electrons feel a stronger attraction to the nucleus and the size of the atom decreases.So as the Atomic size increase so does Ionization Energy. Ionization energy increases across a period and decreases down the family.

Ionization energy is the energy required to remove the most loosely held electron from a gaseous atom or ion. A cation has a smaller radius than its neutral atom because. In carbon, for example, the effective nuclear charge is +4 (6 protons minus 2 electrons in the first shell). Atomic size is the distance from the nucleus to the valence shell. The same trend of atomic radius applies once you divide the table into metal and nonmetal sections. Effective nuclear charge is the net positive charge felt by valence electrons, estimated by (number of protons) - (inner electrons). Atomic radii increase toward the bottom left corner of the periodic table, with Francium having the largest atomic radius.
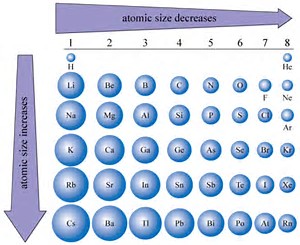 This is because the effective nuclear charge increases with the addition of more protons. An anion is relatively larger in size than its parent atom. The atomic size of a cation will be smaller than that of the parent atom. The Ionic radius can be described as the distance between the nucleus of an ion and the outermost shell of the ion. Second, atomic radius decreases from left to right across a period. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. Electrons in the highest energy level are farthest away from the nucleus, so the size of the electron cloud increases going down the periods. Energy levels are represented by the coefficients when you write electron configurations (ex. To predict relative ionic sizes within an isoelectronic series. The closer the electrons are to the nucleus, the smaller the distance. This means that there is a higher nuclear charge which increases the pull the nucleus has on the electrons. Again, this is due to the effective charge at the nucleus. This direction increases the size of the atom. As you go right, the atomic numbers increase. The other trend of atomic radius or atom size occurs as you move vertically down an element group. The higher the period, the higher the energy level. AP Chemistry Atomic size, Ionic size, Ionization Energy Worksheet. Last updated 8.5: The Explanatory Power of the Quantum-Mechanical Model 8.7: Ions- Configurations, Magnetic Properties, Radii, and Ionization Energy Learning Objectives To understand periodic trends in atomic radii. Going from left to right on the periodic table, the atomic radii get smaller. \) are from Inorganic Chemistry by Shriver and Atkins, 5th edition, Oxford University Press, Oxford, 2010.There are two things you should know about the periodic trend for atomic radius: First, atomic radius increases as you go down the periodic table. As we know by a trend in modern periodic table that across a period the number of valence electrons increase by one but still the atomic size decreases,so.
This is because the effective nuclear charge increases with the addition of more protons. An anion is relatively larger in size than its parent atom. The atomic size of a cation will be smaller than that of the parent atom. The Ionic radius can be described as the distance between the nucleus of an ion and the outermost shell of the ion. Second, atomic radius decreases from left to right across a period. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. Electrons in the highest energy level are farthest away from the nucleus, so the size of the electron cloud increases going down the periods. Energy levels are represented by the coefficients when you write electron configurations (ex. To predict relative ionic sizes within an isoelectronic series. The closer the electrons are to the nucleus, the smaller the distance. This means that there is a higher nuclear charge which increases the pull the nucleus has on the electrons. Again, this is due to the effective charge at the nucleus. This direction increases the size of the atom. As you go right, the atomic numbers increase. The other trend of atomic radius or atom size occurs as you move vertically down an element group. The higher the period, the higher the energy level. AP Chemistry Atomic size, Ionic size, Ionization Energy Worksheet. Last updated 8.5: The Explanatory Power of the Quantum-Mechanical Model 8.7: Ions- Configurations, Magnetic Properties, Radii, and Ionization Energy Learning Objectives To understand periodic trends in atomic radii. Going from left to right on the periodic table, the atomic radii get smaller. \) are from Inorganic Chemistry by Shriver and Atkins, 5th edition, Oxford University Press, Oxford, 2010.There are two things you should know about the periodic trend for atomic radius: First, atomic radius increases as you go down the periodic table. As we know by a trend in modern periodic table that across a period the number of valence electrons increase by one but still the atomic size decreases,so.







 0 kommentar(er)
0 kommentar(er)
